
This is an interactive learning method to do with your friends, family, colleagues and even students to determine what you have learnt from our cancer awareness posters and resources this month.
Quizzes has a number of benefits according to research by Cook and Babon (2016)
Improve communication skills especially in discussions
Builds confidence
Increase engagement
Higher satisfaction score
Helps retain information
Identify gaps of learning
Understand effort needed to master knowledge
Improve high order thinking (analysis, evaluation and synthesis)
Student undergo a self-exploration of their attitudes towards learning
Quiz One
Quiz Two
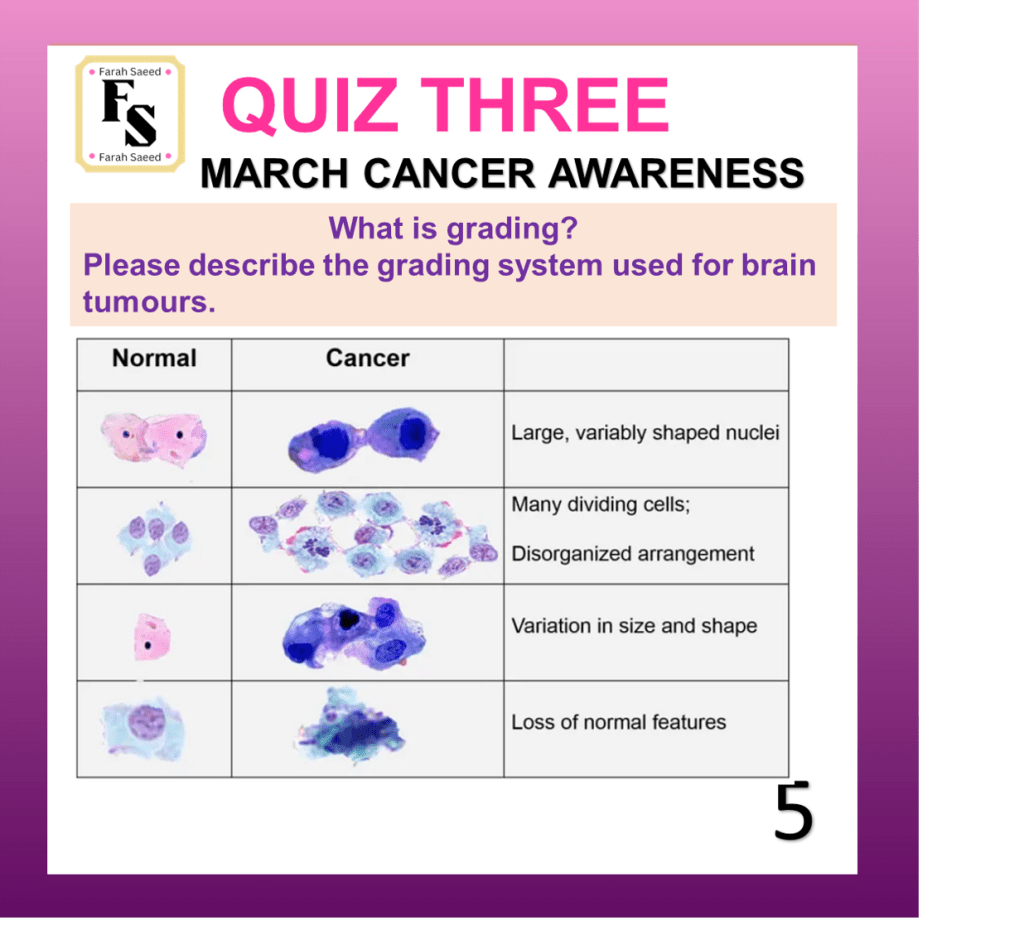
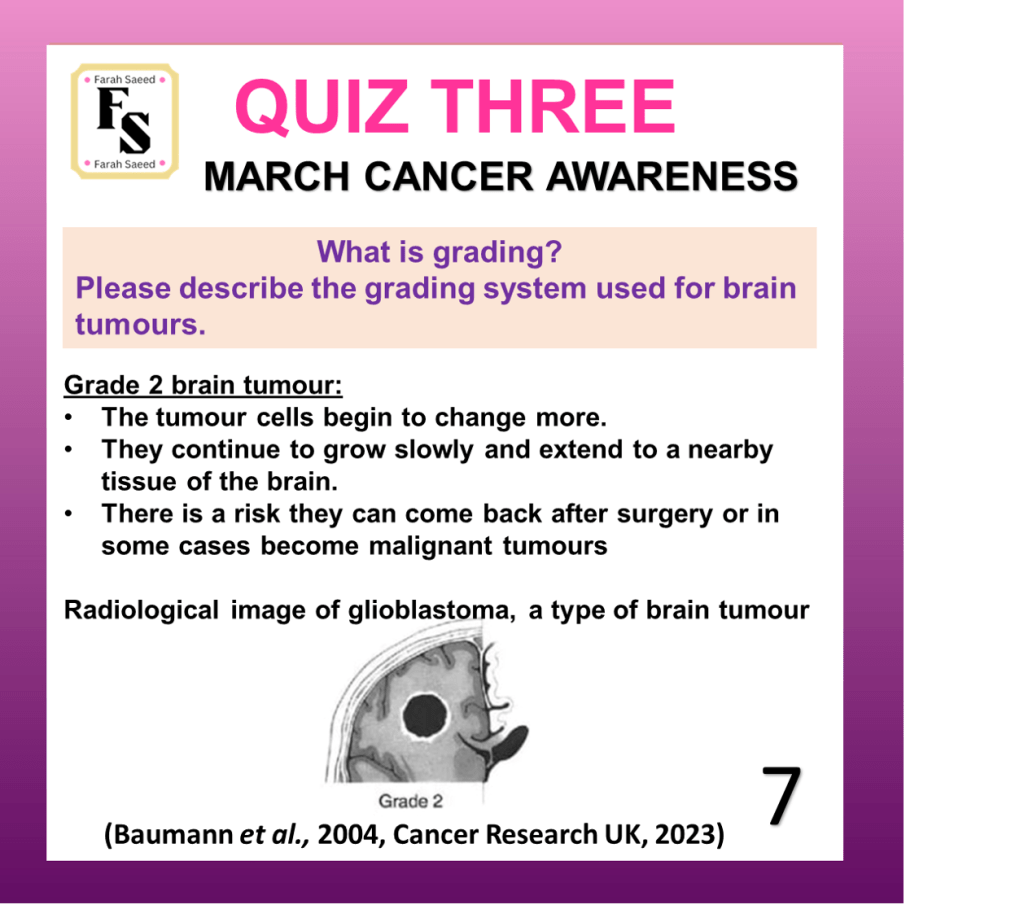
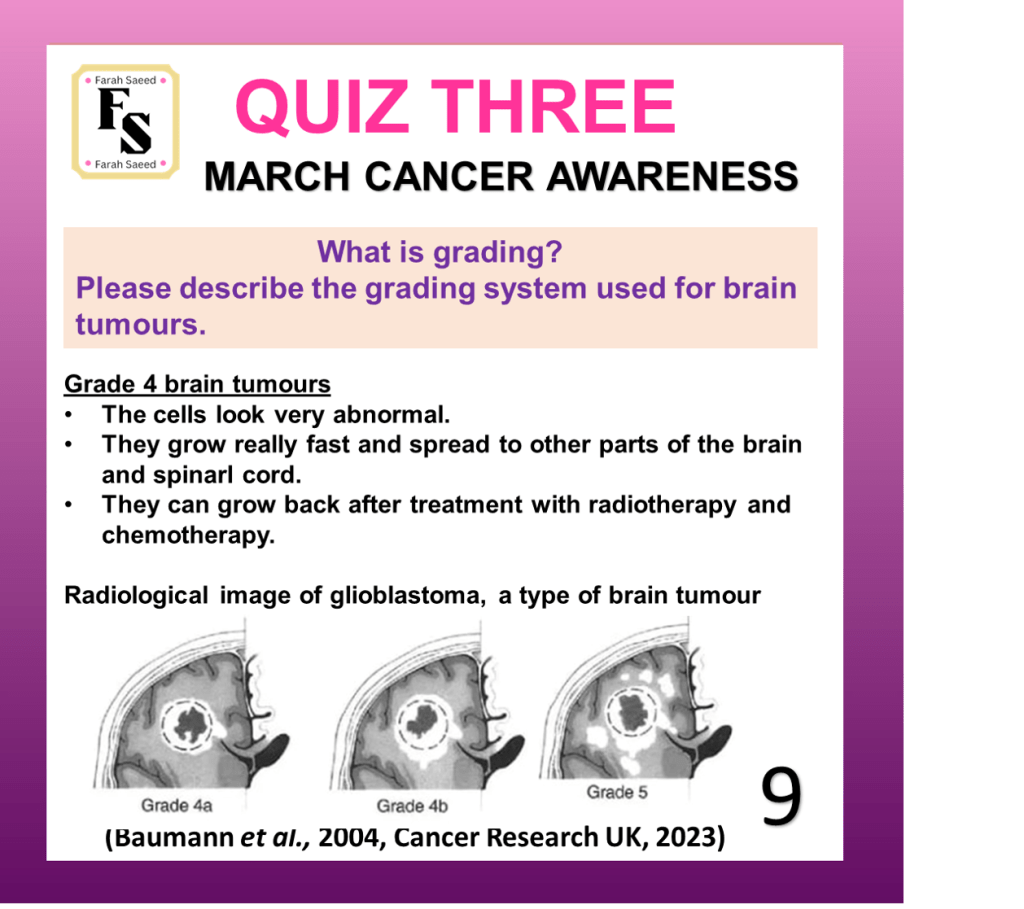
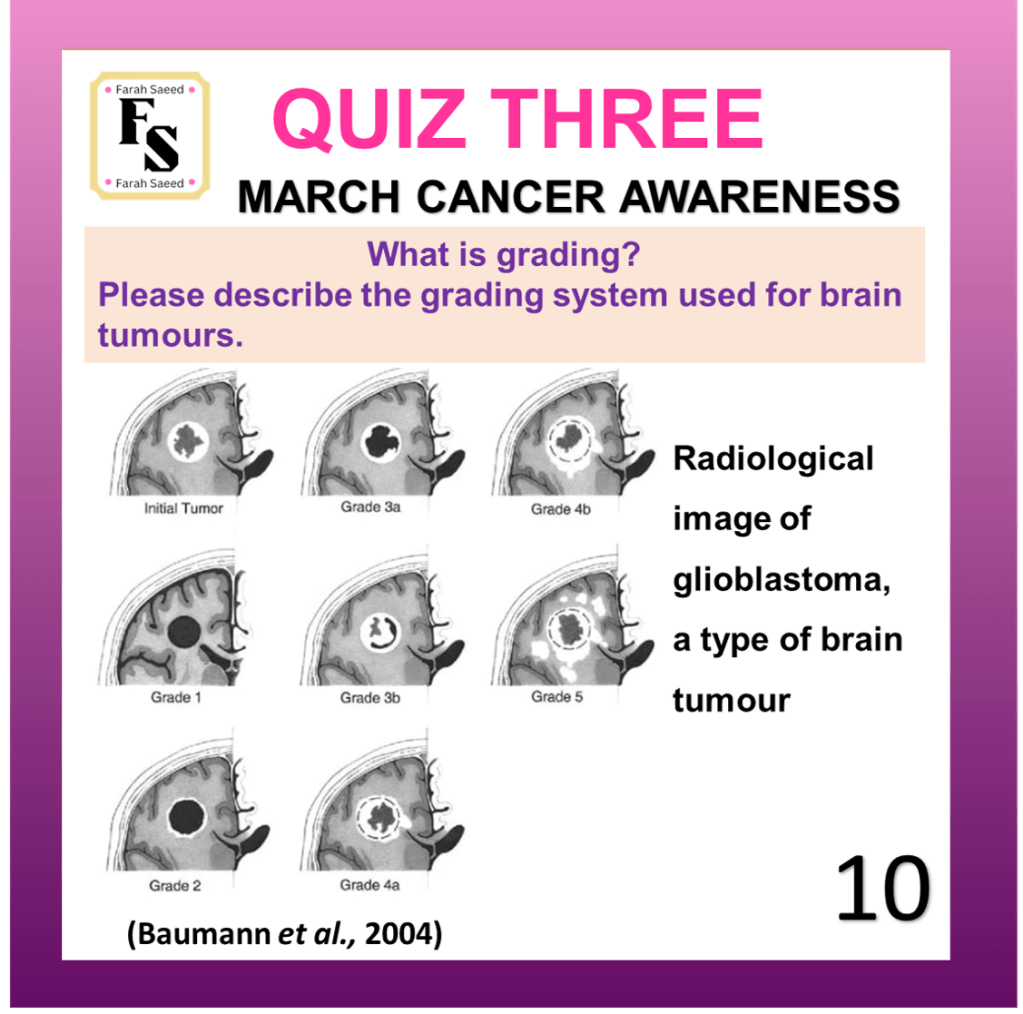
Quiz Three
References for the response of Question Three.
Baumann, F., Miroslava Bjeljac, Kollias, S.S., Baumert, B.G., Brandner, S., Rousson, V., Yasuhiro Yonekawa and Bernays, R.L. (2004). Combined Thalidomide and Temozolomide Treatment in Patients with Glioblastoma Multiforme. Journal of Neuro-Oncology, 67(1/2), pp.191–200. doi:https://doi.org/10.1023/b:neon.0000021803.01170.03.
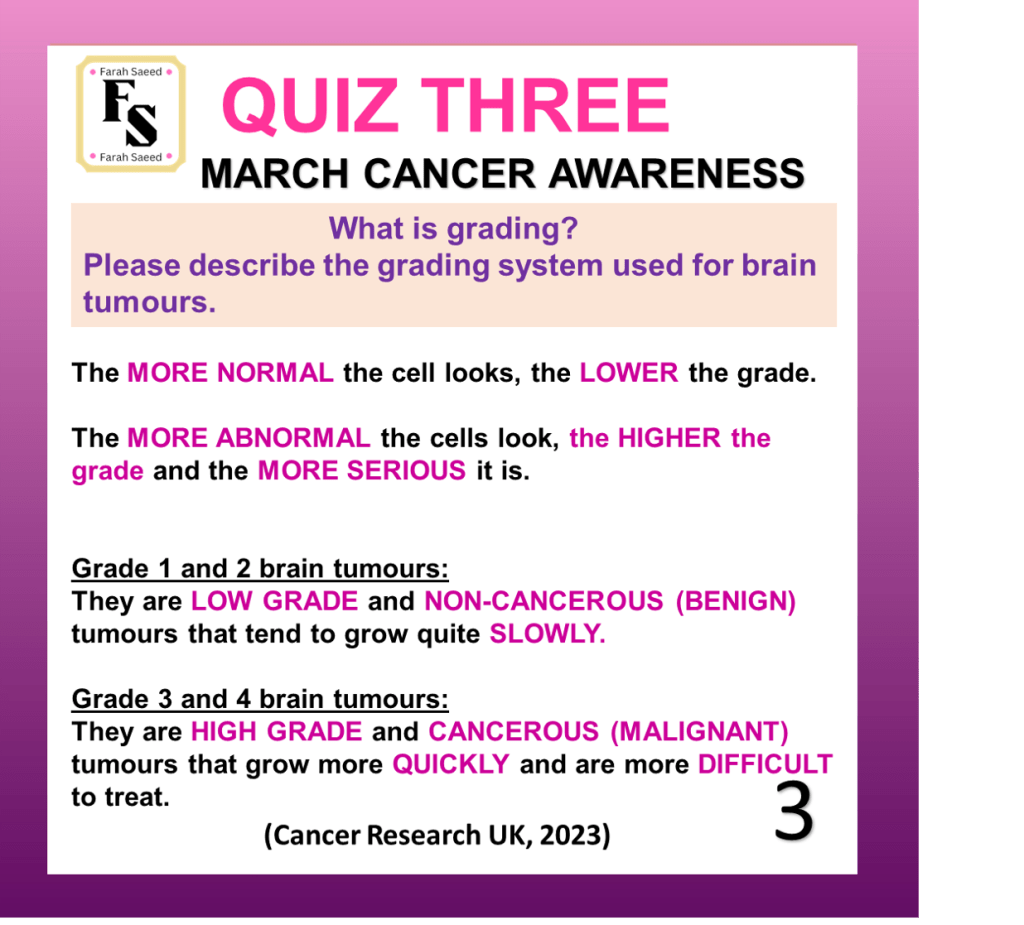
Cancer Research UK (2023) Grades of Brain Tumour. Available at:
https://www.cancerresearchuk.org/about-cancer/brain-tumours/grades (Accessed: 12th June 2025)
Supporting Material for the response of Question Three
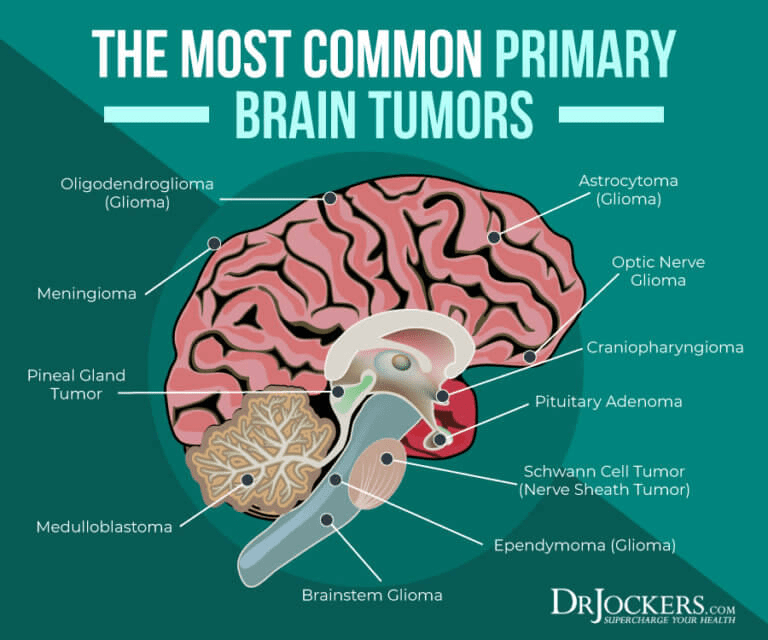
Comprehension One

Please have a read of the article ‘Combined chemotherapy and immunotherapy shows promise for advanced prostate cancers’ published by Dr Heather Buschman from the University of Carolina San Diego in the following link and answer the questions:
- What is chemoimmunotherapy?
- What cancer is investigated in this study?
- Please state three reasons why researchers are focused on this particular type of cancer?
- Why does the cancer cells in this study do not respond to checkpoint inhibitors?
- Why were three difference mouse models of advanced prostate cancer used?
- What did the researchers discover?
- Why are these results from this research study important?
Are you ready to find out how well you did on the questions?
Comprehension Two

Please have a read on Dr Lassman and his team’s research on the effects of Selenoxir on brain cancer patients’ newly diagnosed and recurrent conditions.
- What type of cancer is glioblastoma?
- Many proteins have high expression in solid tumours. What is the name of the overexpressed protein in gliomas, that Selenoxir inhibits?
- What is the function of the protein XPO1?
- How is Selinexor administered?
- What other cancers can Selinexor be used to treat?
- What happens when you inhibit XPO1 via Selinexor?
- Dr Lassmann and his team utilised preclinical mouse models, what effects did they observe?
- What did Dr Lassman and his team discover from their Phase I study of pre-treated patients with metastatic solid tumours (before treatment)?
- How many patients were treated in this study? Please specify how many underwent surgery.
- What were the sociodemographic factors of the subjects (e.g. age, gender, ethnicity, country, socioeconomic status)?
- What was the duration (length of time) of the study?
- There are four arms in this research study (A, B, C, D). Arm A was designed to explore the pharmacokinetics and pharmacodynamics of Selinexor to treat patients with 1 -3 doses of Selinoxor 50 mg/m2 twice weekly for 12 days before cytoreductive surgery for recurrent tumours. What is the difference between Pharmacokinetics and Pharmacodynamics?
- How many patients were in each Arm of the study?
- What was the median Selinexor concentration used in Arm A?
- What pharmacokinetics experiment did patients in Arm A perform?
- What were the two genetic tests performed on Arm A and archival tissue blocks (three doses, treated with 21 days)?
- What was the purpose of performing these genetic tests?
- What was the goal of Arms B, C and D?
- What was the end point of the study?
- Did Arms B, C and D have different dosing schedules for patient not undergoing surgery?
- What is the purpose of RANO?
- Why did Arm B stop their experiment on 23rd March 2015?
- How were the scheduled events following this event changed?
- From the results, what was the concentration of Selinexor as a single agent as the optimal dose (dose that works well at its maximal rate) and efficacy (the desired effect) for patients with recurrent glioma post-treatment?
- Which Arm achieved the highest percentage of achieving the endpoint of the study (6-month progression-free survival rate?
- Which Arm achieved the lowest percentage of achieving the endpoint of the study (6 month progression-free survival rate?
- From the genetics experiment, what were the mutated genes that occurred? How many participants had this mutation? What are their function? Please utilize the article and your own knowledge.
- What was the percentage of patients of different grade adverse events that were related to the treatment (TRAE) of different tumour grades?
- Compare and contrast some of the haematological and non-haematological TRAE of patients with AE in glioma patients in Table 3.
Are you ready to find out how well you did on the questions?
Supporting material for Comprehension Two
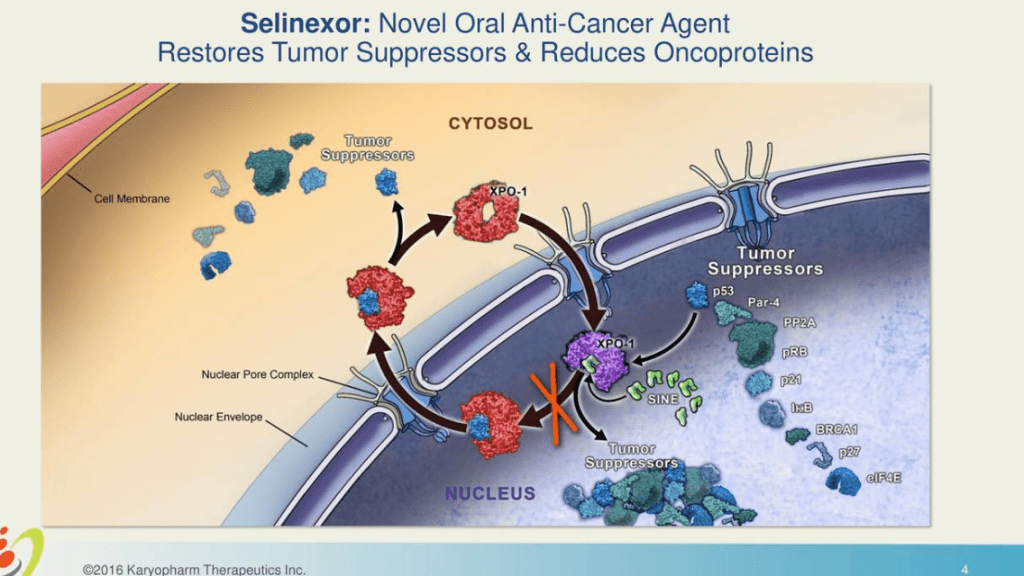
Comprehension Three

Please have a read on Dr Nel Syed and his team’s research on how radiotherapy helps patients with Glioblastoma
https://www.imperial.ac.uk/news/234035/new-treatment-could-boost-radiotherapy-effectiveness/
1. Where did the study take place?
2. What is the name of the amino acid that Dr Nel Syed and his team discovered that would help increase sensitivity to radiotherapy if removed?
3. What is the percentage of glioblastoma cases that can make arginine?
4. Which cancers were more effective in response to ADI-PEG20?
5. What type of cell can also remove the amino acid?
6. What is the function of amino acids?
7. What is the percent rate of overall survival of people with glioblastoma for more than ten years?
8. How is glioblastoma commonly treated?
9. How do you think the removal of arginine can increase sensitivity?
10. What does the team hope to do in the remaining 30% that are unable to make arginine?
Are you ready to find out how well you did on the questions?
Supporting Material for Comprehension Three
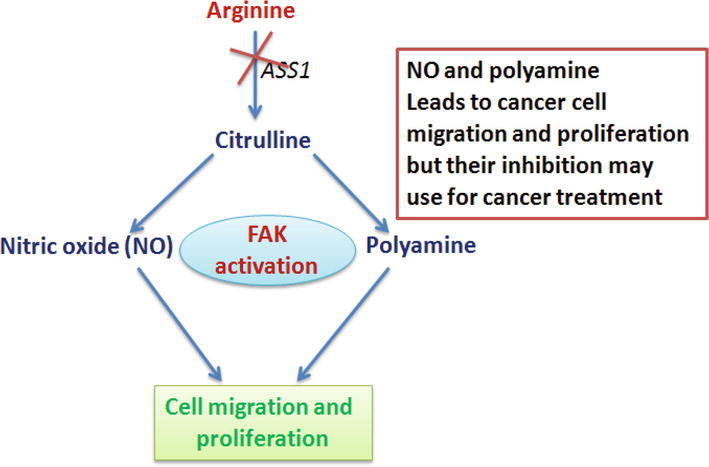
The enzymes (proteins that speed the rate of a chemical reaction) arginosuccinate synthetase1 (ASS1) and arginosuccinate lyase (ASL) of the urea cycle are crucial in the production of arginine before being released into the blood. ASS1 is not expressed in tumours and is a key target in therapy.
Removing arginine via the microbial enzyme, arginine deiminase (ADI) prevents cancer cells from migrating and growing. This is achieved by ADI converting arginine to citrulline and amine group (NH3). In normal cells particularly the liver and kidney, there is high expression of ASS1 and ASL. Therefore, citrulline can be recycled back to arginine.
The drug, ADI-PEG20 consists of a ADI with pegylate. Dr Nel Syed and his team showed anti-tumour activity using in vivo studies in mice and in-vitro studies in cancer cell lines. However, the anti-tumour potential of ADI-PEG20 was observed more in vivo studies.
High levels of nitric acid can activate focal adhesion kinase (FAK) which can assemble the integrin protein. FAK is activated by high levels of nitric oxide (NO). Low levels of ASS1 lead to low production of arginine and less cancer cell migration. In order for cancer cell migration and proliferation to occur, arginine needs two enzymes nitric oxide synthase and arginase1 (Narayan and Kashyap, 2021).
Narayan, K.S. and Kashyap, R. (2021). Arginine Metabolism: An Enlightening Therapeutic Attribute for Cancer Treatment. Available at: doi:https://doi.org/10.5772/intechopen.97254. (Accessed: 15th June 2025)
Updated: June 2025 Review: March 2027






Leave a comment