
Lecture Fifteen: NFKB
This lecture aims to understand the structure and function of the transcription factor, Nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) in cellular processes. To understand how the receptor is activated via extracellular ligands and receptor dimerization. The role of IκB (inhibitory proteins of dimers) and Iκκ proteins was revealed. The IκB forms complexes with NFκB to link the cytoplasm and nucleus inhibiting NFκB. To differentiate the signal transduction of NFκB via canonical and non-canonical pathways. The canonical pathway is stimulated by the Toll-like receptors (TLR), tumour necrosis factor receptors (TNFR), and interleukin-1 receptor (IL-1R) that bound to the ligand. Phosphorylation and degradation of IκB causes the translocation of NFκB to the nucleus. The non-canonical pathway depends on the activation by the TNF receptor family such as BAFFR and RANK. Phosphorylation of NIK phosphorylates IKKa. The p52-RelB heterodimer (subunits of NFKB) translocates to the nucleus for transcription. Awareness of the causes of the dysregulation of the pathway leading to cancer.
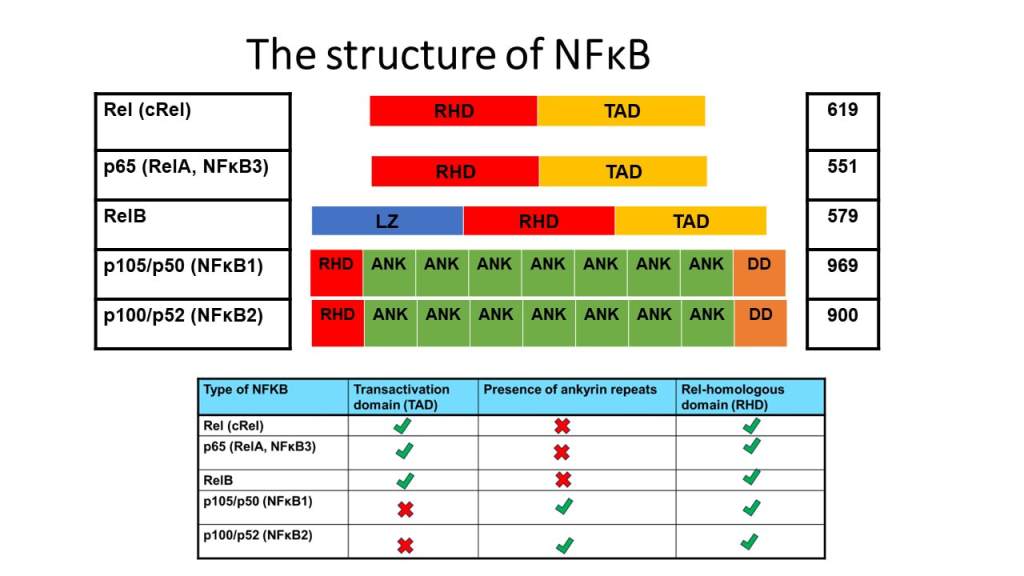
Structure of NFKB
The image presents the structure of the Nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB). The transcription factor has five subunits: Rel (cRel), p65 (RelA, NFκB3), RelB, p105/p50 (NFκB1) and p100/p52 (NFκB2). Within each subunit, several types of domains are distinctive to one another. The Transactivation domain (TAD; light orange) activates transcription of genes. Rel-homologous domain (RHD; red) is where DNA binds, dimerization between the same members and different membranes to translocate into the nucleus. Ankyrin repeats (green) have an inhibitory role. Some types have TAD and RHD whereas, other kinds of NFκB have ankyrin and RHD. Some types of NFκB have a leucine zipper domain (LZ; blue), for instance, RelB that can recognize specific DNA sequences and contains lots of leucine residues. NFκB1 and NFκB2 subtypes have death domains (DD; orange) that promote protein-protein interactions. A larger size of this image is found in the resource list.

Types of IKK
The image presents the structure of IκK that helps transduce the signal inside the cell. There are three subunits: catalytic IκKᾴ, IκKβ, and regulatory subunit IκKγ. IκK-α phosphorylates amino acids present in IκBβ to cause degradation. IκKβ phosphorylates serine amino acid residues of IκBα and IκBβ. IKK gamma regulates IκK activity and interacts with IκKα and IκKβ. IκKα and IκKβ have similar domains but IκKγ is more distinctive. A larger size of this image is found in the resource list.
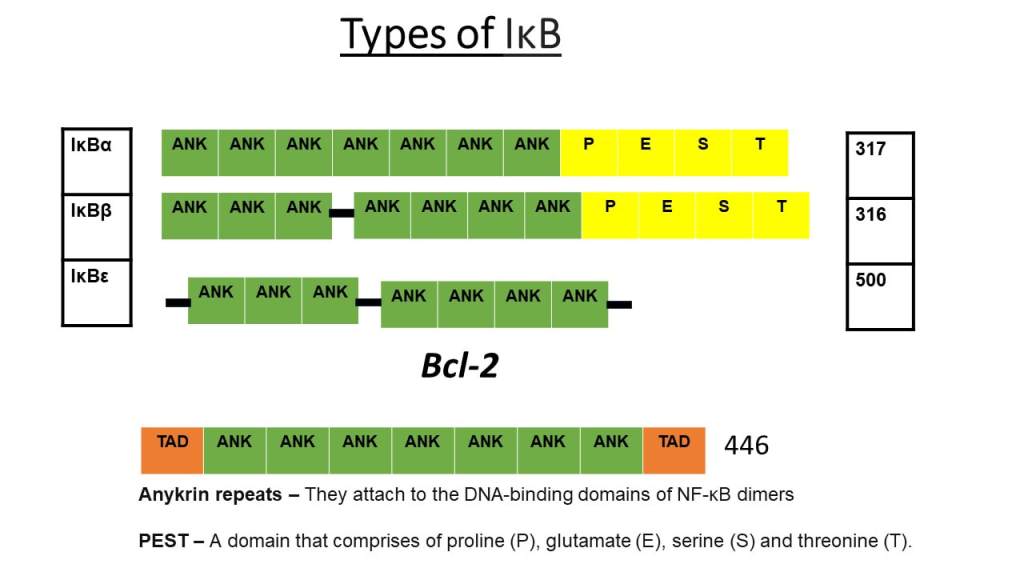
Types of IKB
The image presents the structure of IKB, inhibitory proteins of dimers that transduce signals inside the cell. The three main types are IKBα, which prevents p50/RelA heterodimer. IKBB prevents RelA/cRel heterodimer. IKBε prevents RelA and c rel dimers. Ankyrin repeats (green) reside in all types of IKB. A domain of amino acids: proline, glutamate, serine, and threonine (PEST; yellow) are in IKB-alpha and IKB-beta. IKBε has all ankyrin repeats. Some nuclear IKB inhibit and activate NFKB transcription of genes. A larger size of this image is found in the resource list.
Resource List For Lecture Fifteen
Youtube video
Glossary
Quiz
PDF formats of the images
Leave a comment