Lecture Sixteen: HER2
This lecture aims to reveal the structure and function of one of the family members of the Epidermal Growth Factor Receptor tyrosine kinase family, HER2, otherwise known as erbB2 and HER2/neu. The journey of cellular communication from receptor activation, signal transduction, and cellular response is discussed in the lecture. HER2 has high catalytic activity and is activated by two techniques: Homodimerization and Heterodimerization. Upon binding with HER3 to form a hetero dimer, typical ligands that bind are epiregulin and neuregulin. However, HER2 -HER4 heterodimer can interact with EGF, TGF, Heparin, neuregulin, and betacellulin ligands. This highlights that HER2 does not have a specific ligand and depends on the dimer. Autophosphorylation takes place after dimerization. Signal transduction via STAT kinase, Ras/MEK/MAPK, and Phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) occurs. HER2 induces transcription in the nucleus by interacting with importin 2 to induce a cellular response. Several causes lead to dysregulation of the HER2 signaling factor particularly, overexpression of HER2, mutations in the kinase domain, polymorphism, and deregulation of HER2 genes affecting cellular processes.
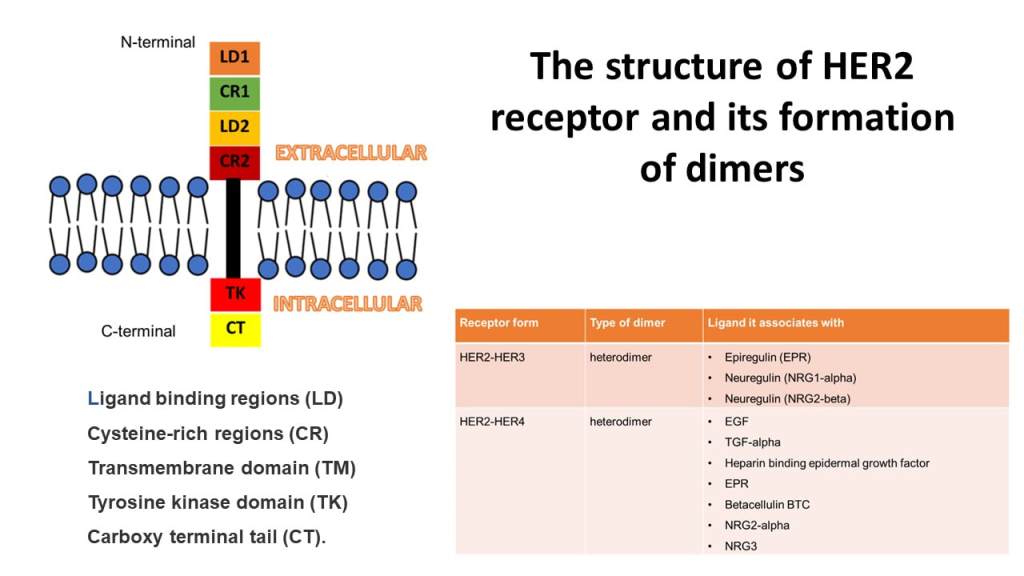
Structure of HER2
The image presents the structure of HER2 (erbB2, HER2/neu) tyrosine kinase enzyme and typ1 transmembrane growth factor receptor. There are three domains: an extracellular ligand binding domain, a transmembrane domain, and an intracellular tyrosine kinase domain. Ligand binding region (orange), and cysteine-rich regions (green and dark red) are found in the N terminal extracellular region. Transmembrane region (black). The Intracellular region contains tyrosine kinase (scarlet red) and carboxyl-terminal tail (yellow) in the C-terminal.
Resource List For Lecture Sixteen
Youtube video
Glossary
Quiz
PDF formats of images
Leave a comment